Natural abundance
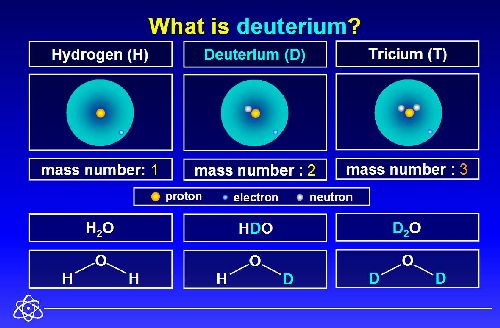
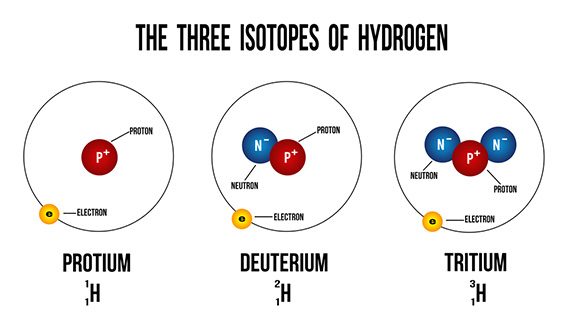
Deuterium occurs in trace amounts naturally as deuterium gas, written as ²H2 or D2. However, most deuterium atoms in the universe are bonded with typical ¹H atoms to form the gas called hydrogen deuteride (HD or ¹H²H).
The abundance of deuterium in the oceans of Earth is approximately one atom in 6500 hydrogen atoms (about 154 parts per million (ppm)). Deuterium thus accounts for approximately 0.015 percent (on a weight basis, 0.030 percent) of all naturally occurring hydrogen atoms in the oceans on Earth; the abundance changes slightly from one kind of natural water to another.The abundance of deuterium on Jupiter is about 6 atoms in 10,000 (0.06 percent atom basis). There is little deuterium in the interior of the sun , because thermonuclear reactions destroy it. However, it persists in the outer solar atmosphere at roughly the same concentration as in Jupiter.
The existence of deuterium on Earth, elsewhere in the Solar system (as confirmed by planetary probes), and in stars (as indicated by their spectra), is an important piece of information in cosmology. Stellar fusion destroys deuterium, and there are no known natural processes (such as cluster decay), other than the Big Bang nucleosynthesis process, which might have produced deuterium at anything close to its observed natural abundance. This abundance seems to be a very similar fraction of hydrogen, wherever hydrogen is found. Thus, the existence of deuterium at its present abundance is one of the arguments in favor of the Big Bang theory over the steady state theory of the universe. It is estimated that the abundances of deuterium have not changed significantly since their production more than 14 billion years ago.
The world's leading "producer" of deuterium (technically, enricher or concentrator of deuterium) was Canada, until 1997, when the last plant was shut down. Canada uses heavy water as a neutron moderator for the operation of its CANDU reactors. Currently, India is probably the world's largest concentrator of heavy water, also used in nuclear power reactors.
Ref: http://www.newworldencyclopedia.org/entry/Deuterium